Applications
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Neuroscience
Parkinson’s disease causes the loss of dopamine-producing neurons in the substantia nigra, an area of the brain involved in movement. Researchers led by Dr. Su M. Metcalfe from the University of Cambridge are pioneering an approach that could make it possible to replace lost dopaminergic neurons with new cells.
One key challenge to successfully replacing lost dopaminergic neurons with healthy cells is figuring out how to promote the long-term survival of transplanted cells. Delivering neurogenic growth factors or small molecules to the cells might offer one solution.
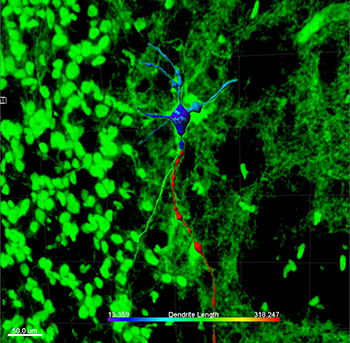
This image shows a transplanted human fetal dopaminergic cell that has differentiated in vivo from a freshly isolated fetal midbrain precursor cell. After coating with stromal nanoparticles, the cell was implanted into the corpus striatum of a nude rat brain. The image was processed using Imaris Filament Tracer software, and the colors relate to the dimensions of neurites and the cell body and are indicative of a mature dopaminergic cell. Other human cells in the graft are shown in green. Courtesy of Su M. Metcalfe.
“Our work pioneers the concept of a surrogate nano-therapeutic stroma to promote and protect human fetal dopaminergic cell grafts in Parkinson's Disease,” explains Dr. Metcalfe. “Our strategy has been to exploit PLGA nanoparticles to create a cell type-specific stromal vehicle for paracrine delivery of cargo”
PLGA is an ideal material for this application since it is already FDA-approved for use in clinical applications such as soluble stitches. To test their approach, the researchers preloaded PLGA nanoparticle-based stroma with neurogenic growth factor and then attached the particles to freshly isolated human fetal brain cells prepared in the same way as for a clinical transplant. Using Imaris Filament Tracer, they processed images of individual cells, which allowed them to visualize the cell body and neurite diameter, size, and shape and thus determine the maturity of the differentiated dopaminergic cells.
Single cell interrogation
“The power of Imaris in the interrogation of a single cell in situ provided unique insight,” says Dr. Metcalfe. “This enabled us to identify the state of differentiation of human primary fetal brain cells - a rare and valuable resource - in a preclinical model of human cell grafts for treatment of Parkinson's disease.”
The researchers found that the number of surviving human fetal dopaminergic cells tended to be higher compared with controls treated with empty nanoparticles. Not only did the nano-stroma help increase cell survival, but in vivo differentiation to dopaminergic cells also increased.
Due to the ethical and practical problems of using fetal tissue, future approaches to cell transplantation are aimed at using embryonic stem cells or induced pluripotent cells to derive dopaminergic cells that can be transplanted into the brain. The success of Dr. Metcalfe’s experiment shows that the same approach might work for Parkinson’s disease cell therapies derived from embryonic stem cells or induced pluripotent cells.
Linking nanotechnology and medicine
“There are very few studies that bring together nanotechnology and medical disciplines as directly as we do here, and, notably, where the potential therapeutic gain is within the major area of neurodegenerative disease,” Metcalfe says. “The analytical imaging of the grafted human dopaminergic cells in situ using Imaris software provided highly sophisticated documentation, confirming successful outcome of a novel nano-medical approach able to synergize with regenerative medicine.”
Author: Su M. Metcalfe and colleagues, University of Cambridge
Category: Case Study
