Applications
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Researchers led by David B. Frank and his post-doctoral fellow, Aravind Sivakumar, from the University of Pennsylvania and Children’s Hospital of Philadelphia, are using Imaris to better understand when lung cells required for gas exchange arise during development. Their findings could help explain why premature infants with undeveloped lungs are able to survive.
Infants who are premature have significantly underdeveloped lungs that can be further damaged after birth. With advances in clinical care, survival of premature infants is improving, and doctors are observing increasingly earlier gestational survival. However, scientists haven’t understood why this is possible because research on lung development suggested that the specialized alveolar type 1 and type 2 (AT1, AT2) epithelial cells required for gas exchange don’t arise until later in lung development.
In the new study, the researchers took a closer look at AT1 and AT2 development using a combination of genetic approaches and cell lineage tracing method to track AT1 and AT2 cells as they arise from progenitors in the lungs of mice. One challenge in studying these cells is that early in lung development, the fetal lung is a tightly compacted structure of endodermal tubes, immature vasculature, and mesenchyme with very small airspaces.
“Defining cell type-specific cellular boundaries in the fetal lung is difficult using conventional imaging and manual analysis in this dense structure,” said Frank. “Imaris 3D surface rendering helped us improve the cellular boundary used to define a cell.”
The researchers also used the Imaris Coloc tool to conduct a new, mostly unbiased, method to define a type 2 alveolar epithelial cells based on the specific intracellular expression pattern in a cell. The tool helped them visualize and quantify colocalized gene expression. They found that cells that could develop into either AT1 or AT2 cells (dual lineage) didn’t significantly contribute to alveolar epithelial population. Instead, it was progenitor cells specified with a single cell type (AT1 or AT2) that contributed to most of the alveolar epithelial cell population.
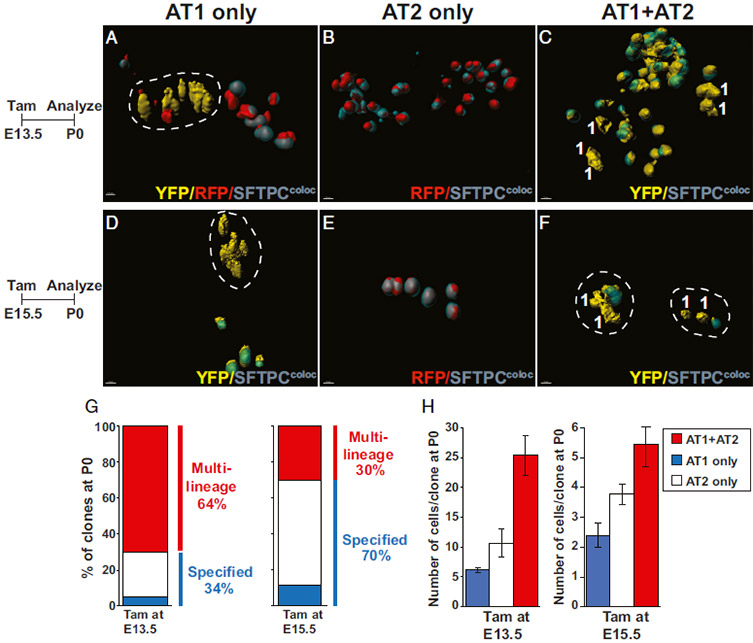
Clonal lineage tracing of Nkx2-1+ lung embryonic alveolar progenitors in thick sections. (A–C) Nkx2.1CreERT2:R26RConfetti pregnant dams were injected with tamoxifen at E13.5, and embryos were analyzed at P0. Analysis revealed single color clones of cells composed of AT1 cells (A), AT2 cells (B), or both cell types (C). (D–F) Nkx2.1CreERT2:R26RConfetti pregnant dams were injected with tamoxifen at E15.5, and embryos were analyzed at P0 (E). Alveolar epithelial clones comprising AT1 cells (D), AT2 cells (E), and both cell types were identified (F). Histology on 150-μm tissue sections counterstained with SFTPC, processed for colocalization with fluorescent proteins, 3D surface-rendering using Imaris, and represented in images as SFTPC colocalization in teal/gray color. Cells were manually highlighted and marked as AT2 cells if they expressed SFTPC and AT1 cells if they did not. (G and H) Quantification of clonal analysis including clone composition and clone size of Nkx2.1+ cells at E13.5 and E15.5 (n = 46 and n = 44 clones, respectively). Mixed AT1/AT2 clones include any combination less than 100% pure AT1- or AT2-only clones. (Scale bars, A–F, 10 μm.)
“The findings from our study revealed that the ontogeny of these specialized cells is much earlier in development than previously thought, which may explain the ability of extremely premature infants to survive,” said Frank. “This study has helped define a critical period of lung development for alveolar epithelial ontogeny and allows us to focus discovery of important molecular players in alveolar epithelial specification.”
The researchers plan to continue using Imaris as they explore the structural morphogenesis that helps instruct cell identity in the lung during development. “The software helps refine tissue relationships in a 3-diminensional fashion that can be applied to not only lung endoderm but also vasculature and the mesenchymal niche,” said Frank.
Research paper: Frank DB, Penkala IJ, Zepp JA, Sivakumar A, Linares-Saldana R, Zacharias WJ, Stolz KG, Pankin J, Lu M, Wang Q, Babu A, Li L, Zhou S, Morley MP, Jain R, Morrisey EE. 2019. Early lineage specification defines alveolar epithelial ontogeny in the murine lung. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1813952116.
Author: David B. Frank and colleagues, University of Pennsylvania and Children’s Hospital of Philadelphia
Category: Case Study
