Applications
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Neuroscience
The precise anatomy of our neuronal network wiring both enables and restricts the functions of the brain. A new tool developed by Matthias Georg Haberl and colleagues makes use of Imaris software to investigate the anatomy and physiology of neural circuits and could help scientists better understand the important link between the structure and function of neuronal circuits.
Seeing detailed neuronal features
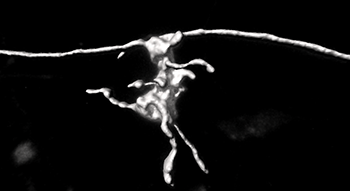
The strong labeling intensity of the anterograde rabies virus allows a fast and reliable reconstruction of fine anatomical features using Imaris. The mossy fiber boutons can have thin elements called filopodia, which are connections to interneurons, and that are evident in this reconstruction.
Haberl and his colleagues developed a new tool that makes it easier to label and then reconstruct the morphologic features of neurons, including the location of the soma, dendrite and axon morphology, and synaptic contact points. Although reconstructions of individual neurons have revealed important insight into the canonical circuit organization of the neocortex, filling single cells with fluorescent dye and manually reconstructing neurons throughout the whole brain is technically challenging and cumbersome.
The researchers engineered a new glycoprotein for the recombinant glycoprotein-deleted rabies virus, rendering the virus as an anterograde vector that can be used to locally infect neurons sparsely or in bulk. The new viral method exploits the strengths of recombinant rabies virus and provides the fluorescence intensity required for computational reconstructions. Single viral particles of the anterograde rabies virus strongly express fluorescent proteins. These fluorescent proteins fully fill all the neuronal processes including axons, which often project several millimeters through the mammalian brain.
The researchers combined their new viral method with Imaris to create 3D reconstructions of entire dendrites, spines and synaptic boutons and to subsequently quantify key anatomical parameters. They also used Imaris Filament Tracer for spine quantifications of neocortical neurons.
“We demonstrated the strength of the vector by computationally reconstructing all key morphological features of neurons: dendrites, spines, long-ranging axons throughout the brain as well as bouton terminals,” said Haberl. “Imaris provided us with crucial information about the surface and volume of dendrites and synapses that let us not only measure dimensions, but also determine complexity. This helped us determine if there were any alterations of contact sites, for example.”
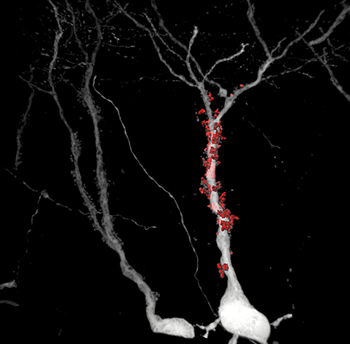
The high signal-to-noise ratio of the rabies virus allowed the researchers to precisely reconstruct these structures, which are the postsynaptic counterpart of mossy fiber boutons.
Studying neurons in aged animals
The new vector works efficiently in many cell types and brain areas. Unlike many other viral tracers, it also works in aged animals. This has allowed the researchers to use their new technique to reconstruct fine morphological details in the aged mouse brain and to study the effect of Alzheimer’s Disease on neuronal morphology.
The researchers have used their anterograde tracer in combination with a retrograde variant to study the effects of Fragile X Syndrome on neuronal connectivity in the visual cortex. They also used anterograde rabies virus mediated tracing in combination with Imaris for 3D reconstructions to study synapse number and morphology in Fragile X Syndrome and Alzheimer’s Disease at an early stage of the disease and in aged mice models. Next they would like to examine the patterns of canonical circuits from and to different areas of the mammalian neocortex.
Related Papers: Haberl MG, Zerbi V, Veltien A, Ginger M, Heerschap A, Frick A. Structural-functional connectivity deficits of neocortical circuits in the Fmr1 (-/y) mouse model of autism. Sci Adv. 2015 Nov 20;1(10):e1500775.
Viana da Silva S, Haberl MG, Zhang P, Bethge P, Lemos C, Gonçalves N, Gorlewicz A, Malezieux M, Gonçalves FQ, Grosjean N, Blanchet C, Frick A, Nägerl UV, Cunha RA, Mulle C. Early synaptic deficits in the APP/PS1 mouse model of Alzheimer's disease involve neuronal adenosine A2A receptors. Nat Commun. 2016 Jun; 17 7:11915.
Author: Matthias Georg Haberl and colleagues, INSERM and University of Bordeaux, France.
Category: Case Study
