Applications
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Markus Sauer and colleagues at the University of Würzburg in Germany are combining a sample preparation approach called expansion microscopy with structured illumination microscopy to make new discoveries about the molecular architecture of the mammalian synaptonemal complex (SC). This meiosis-specific nuclear multiprotein complex is essential for proper synapsis, recombination and segregation of homologous chromosomes.
Although super-resolution microscopy techniques such as dSTORM and PALM have provided new insights into how proteins are organized in cells and multiprotein complexes, structural details of multiprotein complexes are difficult to obtain using these approaches. This is often because of insufficient structural resolution, which is tied to labeling density.
Expansion microscopy offers a way to observe structural details that can’t be seen with super-resolution microscopy approaches. By using a polymer system to physically expand a biological sample, it can bypass the diffraction limit to enable super-resolution imaging with standard fluorescence microscopes.
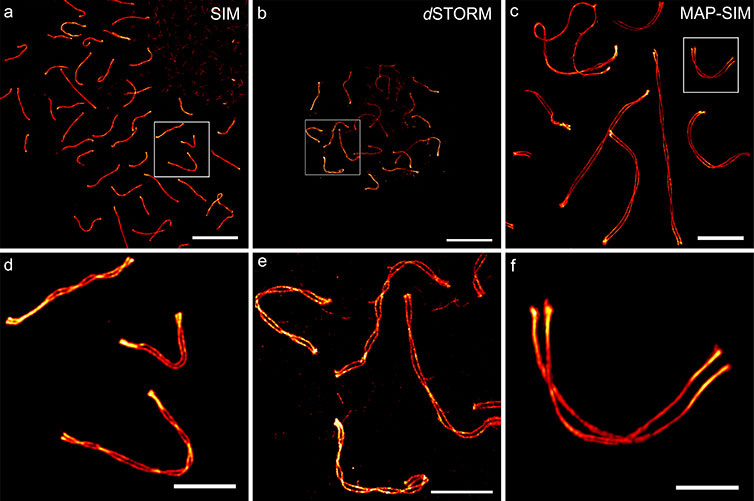
To better understand the best way to apply expansion microscopy for studying the mammalian synaptonemal complex with structured illumination microscopy, Sauer’s research team tested various expansion protocols. For the analysis they used software they developed called LineProfiler that automatically detects fiber-like structures in confocal or super-resolution images. It can be used to analyze protein distributions along biological structures like the SC or microtubule filaments.
“LineProfiler sets pixelwise cross-sectional profiles along the course of the fibers and fits the measured intensity distribution,” said Sauer. “By calculating an averaged protein distribution curve for each filament and providing an overall averaged intensity profile, the software can be used to automatically analyze protein distributions and quantities of biological structures.”
In the new study, the researchers applied Imaris to visualize the 3D distribution of SC proteins in images obtained using structured illumination microscopy with the expansion microscopy method known as magnified analysis of the proteome (MAP). MAP expands entire organs, making them four times as large while conserving the overall architecture and 3D proteome organization. Both the fine subcellular details of proteins and organ-scale intercellular connectivity are preserved with this approach.
After MAP expansion, the researchers immunostained the samples prior to imaging. The 3D movies acquired with Imaris allowed them to visualize the transverse filament and central element of the SC. The images revealed a multilayered organization of the central element protein SYCE 3 and the transverse filament protein SYCP 1.
Overall, the study showed that combining MAP with structured illumination microscopy provided better results than the other expansion techniques studied. This combination enabled reliable three-color super-resolution microscopy of SCs from an entire set of spermatocyte chromosomes with a 20 to 30 nanometer spatial resolution. The researchers found that post-expansion labeling improved immunolabeling efficiency, allowing them to discover previously hidden details of the molecular organization of SCs.
“In our next steps, we will try to further improve the resolution of the method to the molecular scale level by combining expansion microscopy with single-molecule localization microscopy using dSTORM,” said Sauer. “We’ll likely continue to use Imaris for analysis in our studies.”
Date: Sep-20
Author: Markus Sauer and colleagues, University of Würzburg
Category: Case Study
