Applications
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Neuroscience
Research has shown that the adhesion proteins known as neuroligins(Nlgs) likely play an important role in establishing fully functional neuronal circuits. Mutations in human neuroligin genes have been linked to cognitive diseases such as autism. The precise role these proteins have in synapse formation, maturation, and maintenance is not yet fully understood. A contributing factor to this mystery is the difficulty associated with studying Nlgs in mice.. Besides the sheer number (in the trillions) of synaptic connections in the mouse brain, rodents have three Nlg genes; this means that single-gene knockout animals compensate with a redundant gene set.
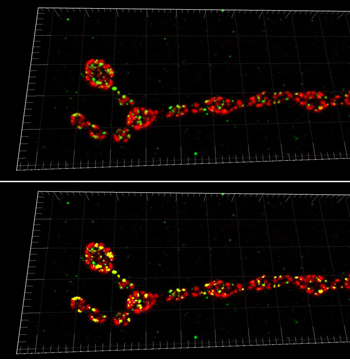
To unravel the in vivo mechanisms of synapse formation, researchers led by Dr. Hermann Aberle of the University of Münster and Dr. Stephan J. Sigrist from the Freie Universität Berlin, both in Germany, examined Nlgs in Drosophila rather than mice. They turned to the extensively studied neuromuscular junction of Drosophila to dissect Nlg function, specifically examining Drosophila Neuroligin 1 (DNlg1), the only muscle-exclusive Nlg in Drosophila.
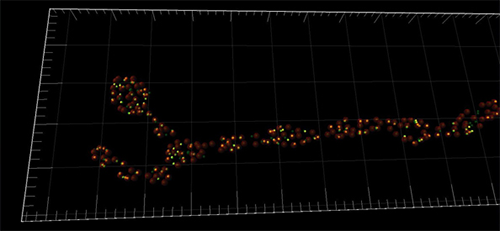
Imaris assisted the researchers with their copious amount of imaging data, mostly in the form of confocal z-stacks. Imaris was used to render the datasets in 3-D and analyze their data using the software’s built-in tools and automated options.
“In our previous work we were often confronted with the quantification of synaptic elements. Counting up to 500 single synapses per neuromuscular junction can be tedious when one does it by hand,” said Omid Khorramshahi, one of the PhD students conducting the study. The built-in spot detection algorithm in Imaris simplified the analysis procedure; the researchers used it to determine the number of synapses in dnlg1 mutants and control animals.
Addtionally, the researchers quantified the colocalization of DNlg1 spots with a postsynaptic marker (GluRIID) by creating a colocalization channel with the ImarisColoc tool, which is part of the software’s optional analysis package. Running the software’s spot detection function in conjunction with the colocalization channel allowed for detection and quantification of colocalization events.. With only a modest amount of work, said Khorramshahi, they used the spot function counts of DNlg1, postsynapses, and colocalization events to calculate the rate of colocalization.
Their analysis showed that mutations in DNlg1 reduced functional synaptic connections by 50% (from ~500 to 240), and lack of DNlg1 caused severe deficits in postsynaptic differentiation. Certain individual active zones – the part of the presynaptic plasma membrane where synaptic vesicles dock and fuse – and some entire boutons lacked postsynaptic glutamate receptor fields. When overexpressed in the muscle, Nlg triggered prosynaptic differentiation at innervations which normally lack these specializations. Overall, their data showed that DNlg1 should not be seen as an absolutely essential organizer of synapse formation, but more as a regulatory factor of postsynaptic differentiation.
Research Paper: Drosophila Neuroligin 1 Promotes Growth and Postsynaptic Differentiation at Glutamatergic Neuromuscular Junctions, Neuron 66, 724–738, June 10, 2010, DOI 10.1016/j.neuron.2010.05.020.
Author: Omid Khorramshahi, Freie Universität Berlin, and colleagues
Category: Case Study
