Applications
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Visualizing the complex 3D vascular architecture of mouse brains after a stroke
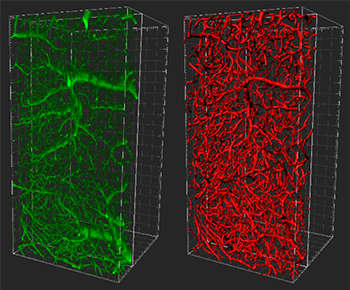
Left (green): Reconstruction (blend mode, gamma 3) of microvessels from healthy brain region in the striatum adjacent to cortex. Right (red): Filament tracer Tracer model. Large divisions on frame represent 50 microns. Courtesy of Anthony Squire, IMCES: imaging facility at the University Clinic Hospital.
Researchers from the laboratories of Prof. Dirk Hermann, Prof. Andreas Faissner and the IMCES imaging facility of Prof. Matthias Gunzer, Germany, have combined optical clearing, lightsheet microscopy, and Imaris software to visualize the complex 3D vascular architecture of mouse brains after a stroke. This work represents the first time that optical clearing and lightsheet microscopy have been used to quantify blood vessel damage by stroke and is a first step towards understanding how blood vessels breakdown after stroke cuts off blood flow to an area of brain.
Using their new imaging approach, the researchers built a 3D model of the blood vessels from which various metrics can be extracted to quantify the degree of vascular integrity or damage after stroke. “This 3D model also offers the potential for a more accurate and reliable assessment of therapeutic agents, either with regards to their beneficial effects as inhibitors of blood vessel breakdown or as stimulators for new vascular growth and remodelling after stroke,” said Dr. Anthony Squire, Head of the imaging facility at the University Clinic Hospital and member of the research team.
Dense network of vessels
The brain, especially the cortex and surrounding regions, features a dense network of blood vessels that bring a constant supply of oxygen and nutrients while also removing metabolites. After a stroke, damaged vessels tend to degenerate, bringing negative consequences for the surrounding tissue and leading to loss of motor and cognitive abilities.
The research teams developed an improved way to visualize and quantify the damaging effects of stroke on the vascular architecture of intact whole mouse brains. The imaging approach uniquely combines previously reported techniques while incorporating several new optimization steps.
To image mouse brains after stroke, the researchers induced stroke using a method that produces a controllable and localized infarct in the striatum region of one brain hemisphere. This enabled the matching region in the healthy brain hemisphere to be used as a comparative control.
To fluorescently label the vasculature, the researchers transcardially perfused a mixture of FITC-conjugated albumin and liquid gelatin into the mice. The mixture quickly solidifies within the vasculature, filling the vessels with a solid, long-lasting fluorescence label with high-signal contrast. “Having a solid fluorescent label, in comparison to the hollow tubes obtained with endothelial markers, was particularly important for the successful 3D modelling of vessels using the Imaris Filament Tracer,” said Squire.
The researchers then used an optimized adaption of the 3DISCO tissue clearing technique to enable whole brain imaging with a LaVision BioTec lightsheet ultramicroscope. After preprocessing the data to further enhance the contrast of vessel-like structures, the researchers turned to Imaris for 3D visualization and filament modelling. One reason the researchers used Imaris was its convenient and easy-to-use interface for reconstructing and manipulating 3D data sets.
Reconstructing 3D vasculature
The researchers used Imaris to reconstruct the vasculature from the initial whole brain imaging data, allowing them to locate the vascular damage caused by the stroke relative to the overall brain architecture. The researchers then imaged this damaged area and its matching site in the healthy brain hemisphere at higher resolution. The next step was to exploit the Imaris Filament Tracer module to model and quantify the complex networks of microvessels in the regions imaged at high resolution.
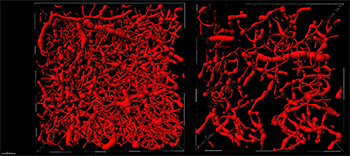
Views looking down on the Filament model from a 200-micron slice taken from a healthy brain region (left) and matching stroke region (right). Major frame divisions represent 100 microns. Courtesy of Anthony Squire, IMCES: imaging facility at the University Clinic Hospital.
According to the researchers, when modelling the architecture of the brain blood vessels, it was important to use the Filament Tracer module in a mode that allowed for filament loops. The parameters for the Filament model could then be established based on a representative data set from a healthy brain region. These parameters formed the basis for batch processing the remaining data sets.
Using Filament Tracer, the researchers created 3D models of the blood vessels in 1 mm3 volumes of the healthy and stroke-affected parts of the brains. Measurements revealed vessel lengths of 922 mm for every mm3 for the healthy brain compared to 329 mm in the stroke affected part of the brain.
“From the Filament models, we also extracted the vessel diameters from which we calculated a vessel length density for three general classes of blood vessels within the measurement volume,” said Squire. “This classification was important for our study since we could confirm our initial observations that the loss of vascular structure in the stroke region was primarily targeted to the capillary blood vessels.”
The researchers continue to work on a number of projects involving the measurements of cleared tissues with a lightsheet ultramicroscope. “In general, Imaris is the software of choice for the visualization and quantification of 3D data sets at the imaging facility,” said Squire. “We, therefore, maintain a full and updated version of Imaris on our high-end workstation.”
Author: Dirk M. Hermann, Dept. of Neurology, University Hospital Essen; Matthias Gunzer, IMCES imaging facil
Category: Andor Academy
