Applications
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Ulrike G.K. Wegst and colleagues at Dartmouth College are using Imaris to study biopolymer scaffolds that can be implanted into living systems, where they can interface directly with tissues and cells to encourage regenerative growth.
“Our goal is to be able to accurately and precisely understand the structural features of scaffolds, so that we may discover through subsequent in vivo studies, which materials perform best and why,” said Wegst. “With such knowledge, we can develop next-generation biomedical materials for clinical applications ranging from peripheral nerve repair to porous ureteral stents.”
Fast, automated analysis
Wegst and her colleagues have developed unique scaffolds that feature a porous microstructure resembling that of natural biological materials. This architecture is created using an ice-templating process known as freeze casting.
Quantitative characterization of scaffold microstructures is typically performed manually by examining scanning electron micrographs. This method is labor intensive, subjective, and can only be used on small areas. It is difficult to automate structural quantification because of scanning electron microscopy’s high depth of field. To overcome these challenges, the researchers developed a method that uses Imaris software for automated, rapid analysis of scaffolds imaged with confocal microscopy, which has a lower depth of field than electron microscopy.
They used the new method to study the effects of water and hydration on the scaffold's micro-architecture. Because living systems are inherently moist it is important to understand how water affects the scaffolds.
“Imaris is not only an excellent tool for biological applications but also very powerful for quantitative analysis of structures in materials science, making it ideally suited for structural quantification of our biomimetic materials,” said Wegst. “We utilized the Cells, Surfaces, and Filaments modules to quantify structural features such as pore area, pore axes, and cell wall thickness.”
Analyzing the scaffolds porous microstructure
The researchers applied the Imaris Cell module to confocal images of the scaffolds. They used cell-only detection because the cell-like pores do not contain nuclei and applied cell boundary detection to identify the cell walls. Starting with the default cell intensity, they adjusted the quality threshold until most pores were correctly segmented. Any pores that weren’t completely in the image were filtered out with a built-in function, and visual inspection was used to correct any incorrectly segmented pores.
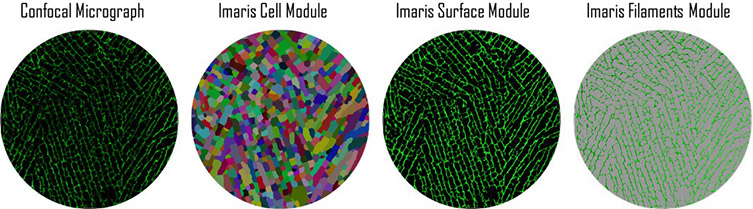
The researchers used the Cell, Surfaces, and Filaments modules to quantify structural features such as pore area, pore axes, and cell wall thickness. Courtesy of Prajan Divakar, Kaiyang Yin, and Ulrike G.K. Wegst, Dartmouth College.
The researchers calculated the number of pixels for each pore and object-oriented long and short axes with built-in software functions. They also calculated the pore aspect ratio by dividing the long axis by the short axis. To determine the pore area without the cell wall, the researchers used the Surfaces module to create a mask based on absolute intensity, which was then subtracted from the original demarcated image. They also used the Filaments module to approximate cell wall thickness by analyzing a mask of a representative confocal micrograph.
Overall, the analysis revealed that, as expected, fully hydrated samples had significantly lower properties than their dry counterparts.
“In addition to the analysis of porous materials, such as the tissue scaffolds, we are also using Imaris to characterize structural surface features at the microscale that we observe in new materials under development in our lab,” said Wegst. “These surface features, like the pore structure, are critical for the in vivo and regenerative performance, and determine the host response at the genetic level.”
Author: Prajan Divakar, Kaiyang Yin, and Ulrike G.K. Wegst, Dartmouth College
Category: Case Study
