Applications
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Neuroscience
Scientists led by Dr. Joel S. Pachter from the University of Connecticut Health Center recently analyzed the brain’s choroid plexus compartments in three dimensions with Imaris software. Linking this information with gene expression data provided a holistic picture of the choroid plexus’s role during neuroinflammation as well as the molecular players involved.
The brain’s choroid plexus structure is the source of cerebrospinal fluid that bathes the central nervous system (CNS). However, scientists have more recently begun to think that the choroid plexus might also be a portal of entry for immune cells to invade the CNS and initiate neuroinflammatory diseases such as multiple sclerosis. A better understanding of the mechanisms involved in this process could allow the development of genetic and pharmacologic therapies that effectively and safely disrupt the pathogenic processes.
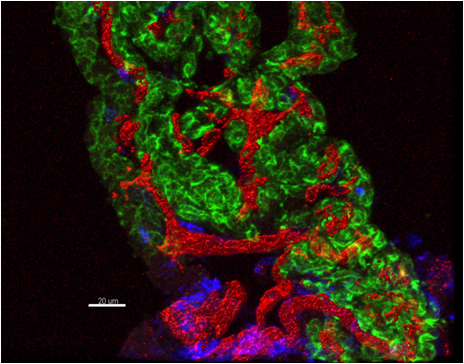
The complex three-dimensional (3D) anatomy of the C57BL/6 mouse choroid plexus during neuroinflammation. The choroid plexus is shown after the onset of clinical EAE following immunization with myelin oligodendrocyte glycoprotein peptide 35-55 (MOG35-55). Seen are the two major choroid plexus compartments: the inner capillaries (red), which become swollen during EAE, and the outer choroidal epithelium (green). Also observed are invading leukocytes (blue) that mediate the inflammatory disease. The image was generated using Imaris based isosurface rendering of 3D reconstructed confocal z-stack derived from an immunostained 60-micron thick mouse brain cryosection. Green: Anti-pan Cytokeratin (epithelial marker) to identify the choroidal epithelial cells. Red: Anti-CD31 (endothelial marker) to identify the stromal capillary plexus. Blue: Anti-CD45 (leukocyte marker) to identify any invading immune cells. Image courtesy of Dr. Joel Pachter.
The researchers are using the experimental autoimmune encephalomyelitis (EAE) animal model of multiple sclerosis to study the choroid plexus. They determined the gene expression patterns in the separate choroid plexus compartments in situ using laser capture microdissection and global RNA profiling. They turned to Imaris for the 3D morphological analysis because they had previously used it in a study that quantified capillary density and extent of branching within cerebral microvasculature.
Examining choroid plexus compartments
For the new research, the investigators used Imaris to create 3D isosurface renderings from confocal microscopy z-stacks of immunostained capillary plexus and choroidal epithelial cells in 60-micron thick brain cryosections. This allowed them to acquire qualitative and quantitative information about how choroid plexus tissue responds to different immune stimuli. “The 3D isosurface rendering provided a detailed perspective regarding the complex arrangements of the different choroid plexus compartments, e.g. the highly tortuous capillary plexus and the adjoining epithelial layer, and helped in visualizing how these are altered during neuroinflammation,” Dr. Pachter says.

Imaris Vantage Gallery plot. In the Gallery panels, the position of the surface rendered inner capillaries corresponds directly to the increased value of the objects Area. Surfaces are color coded according to the position within the image.
To better understand the specific effects of the multiple immune stimuli associated with eliciting EAE, the researchers analyzed capillary diameters within the choroid plexus with Imaris FilamentTracer. They traced the outline of choroid plexus microvessels and acquired the capillary diameters for each 3D dataset from 60-micron immunostained tissue sections in response to different treatments.
“By complimenting the investigation of local, cell-specific patterns of expression of immune related genes with Imaris based morphological analysis of the choroid plexus tissue compartments during evolving neuroinflammation (e.g., using EAE as a model), we were able to identify some key molecular players that mediate disease and can be exploited as novel therapeutic targets,” Dr. Pachter says. He adds that the 3D analysis approach they used to measure microvessel diameter should be readily adaptable for evaluating the changes to brain parenchymal vessels during neuroinflammation and other vascular pathologies in peripheral organs.
Next steps
Having previously used Imaris to study the anatomical and functional heterogeneity in endothelial tight-junction protein expression among the CNS microvessels during neuroinflammation, the researchers are now interested in using Imaris for similar applications with the choroid plexus. The tight junction proteins of the choroid plexus choroidal epithelium could become disrupted during inflammation, thereby allowing immune cells to cross this otherwise intact barrier.
“Imaris provides key 3D image visualization and analysis tools that can be used to image and quantify these changes in tight junction proteins within the epithelial layer during development of disease, as well as evaluate accompanying changes in the choroid plexus capillary plexus,” Dr. Pachter says. “Given the tortuosity of the choroid plexus capillaries, 3D rendering options provided by the software will facilitate image acquisition and more accurate quantification.”
Research Paper: Murugesan N, Paul D, Lemire Y, Shrestha B, Ge S, Pachter JS. Active induction of experimental autoimmune encephalomyelitis by MOG35-55 peptide immunization is associated with differential responses in separate compartments of the choroid plexus. Fluids Barriers CNS. 2012 Aug 7;9(1):15. doi: 10.1186/2045-8118-9-15.
Author: Dr. Joel S. Pachter and colleagues, University of Connecticut Health Center
Category: Case Study
